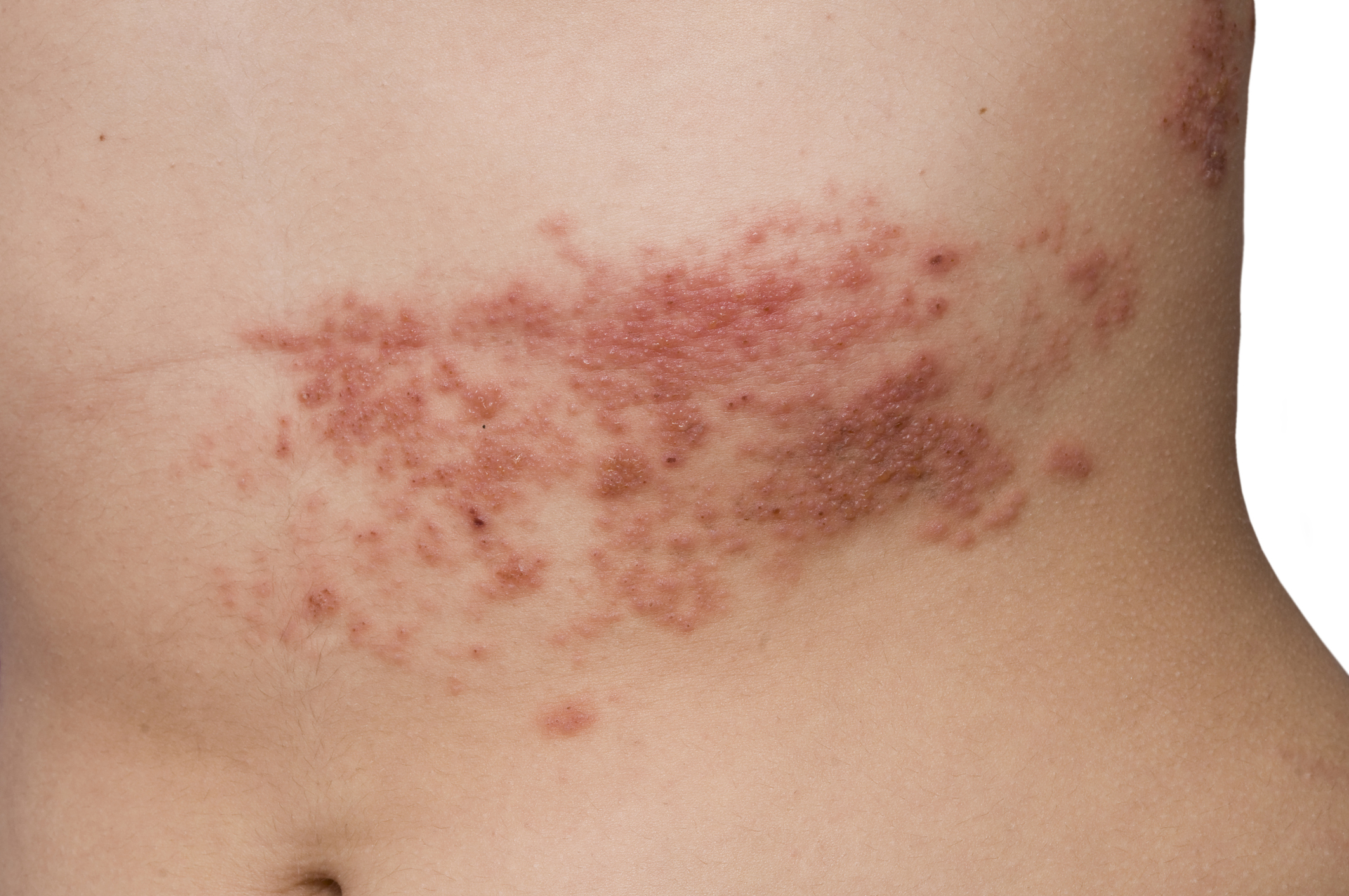
WASHINGTON — Local doctors are involved in clinical trials for a promising new drug to treat the chronic pain often associated with severe cases of shingles.
The goal is to prevent the disabling postherpetic neuralgia — the debilitating pain that can last long after the telltale shingles rash disappears.
“There is nothing on the market today that has been shown to prevent that disabling, burning electrical pain that many folks suffer,” says Dr. Stephen Minton, an internist in Alexandria, Virginia, who is conducting a trial through Alexandria Clinical Research.
Participants in the clinical trial are patients who just developed shingles. They get the new medication within 72 hours of the appearance of the telltale rash, and are then monitored to see if the drug lowers their risk of persistent pain.
Minton says developing these drugs has become the focus of research on shingles — a reactivation of the chickenpox virus in the body that can result in damage to nerve fibers.
“We’re working now to find solutions for that problem,” he says, adding that a new medication for postherpetic neuralgia could revolutionize the treatment of the disease.
“If we can prevent that, that will make a sea change in the amount of disability that we get from shingles,” Minton says, noting chronic pain is the worst complication of the disease.
He is testing a drug from the big pharmaceutical company Novartis, which has worked well in earlier trials.
The National Institutes of Health is also involved in research on shingles, and the Centers for Disease and Prevention has been keeping tabs on the prevalence of the disease.
The CDC says one out of every three Americans will develop shingles at some point in their lifetime, and there are about one million cases each year. And while children and young adults can get it, about half of all cases occur in men and women who are at least 60 years old.