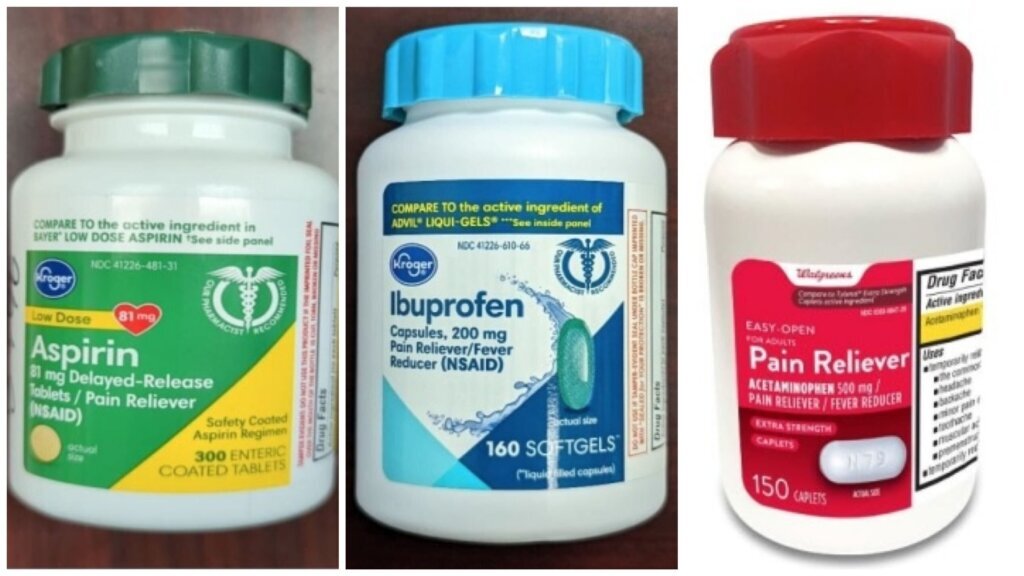
Over 400,000 bottles of medicine sold at Kroger’s and Walgreens locations across the country are being recalled due to concerns about child safety.
The Consumer Product Safety Commission said Thursday that over-the-counter store brands of ibuprofen, acetaminophen and aspirin failed to meet federal standards for child-resistant packaging.
There is no issue with the medicine itself, but the federal regulator warned that a child could be poisoned if they opened the bottle due to the substandard safeguards. No injuries have been reported.
The recall involves Kroger’s 300-count aspirin bottles and 160-count ibuprofen bottles, as well as Walgreens’ 150-count acetaminophen pain reliever bottles.
For a list of specific UPC and lot numbers impacted, see the CPSC’s Kroger and Walgreens recall alert.
Consumers with a recalled product should immediately move it to a safe location out of reach and sight of children, and contact their retailer for a full refund.








