WASHINGTON — A specific batch of Children’s Advil Suspension Bubble Gum, a liquid form of the medicine, is being voluntarily recalled over concerns that a mislabeling could lead to overdoses.
According to Advil manufacturer Pfizer, certain bottles of the popular, over-the-counter pain and fever reliever for children were packaged with the wrong dosage cup.
While recommended dosages were listed in milliliters on the label as normal, the included cup was marked in teaspoons.
Since a single teaspoon is about five times more than a milliliter, Pfizer is worried that using the incorrectly labeled cup could lead to overdoses.
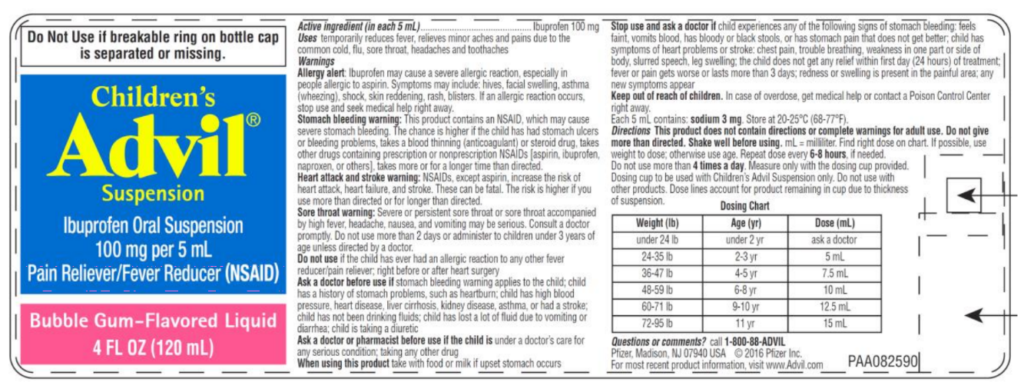
The affected batch includes 4 fl. oz. bottles with lot number R51129 and an expiration date of “11/20”. It was distributed to stores nationwide between May and June 2018.
Pfizer said it had notified distributor and retailers to stop sale of the recalled product.
It said consumers with questions or concerns should contact Pfizer’s information line at 1-800-88-Advil, which operates weekdays between 9 a.m. and 5 p.m.
Adverse reactions can also be reported to the U.S. Food and Drug Administration online at FDA.gov.
Symptoms of ibuprofen overdose include nausea, vomiting, headache, drowsiness, blurred vision and dizziness.
The bottle’s label is shown below: