WASHINGTON — The drug company Allergan voluntarily recalled 170,000 of the sample packs of the birth control pill Taytulla because women could have unintended pregnancies.
The company said their was a packaging error, according to a news release posted on the Food and Drug Administration’s website.
Allergan said four maroon placebo capsules were placed out of order in the sample blister packs of 28 pills handed out by doctors.
The Taytulla pill pack has 24 “active” pink softgel capsules, with hormones, with “WC” printed on the outer shell in white to be taken for 24 days followed by four maroon softgel capsules, without hormones, also imprinted with WC on one side to be taken for the next four days.
If the pills are taken out of order, the contraceptive might not work.
The company said that “the first four days of therapy had four non-hormonal placebo capsules instead of active capsules.”
“As a result of this packaging error, oral contraceptive capsules, that are taken out of sequence, may place the user at risk for contraceptive failure and unintended pregnancy. The reversing of the order may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order,” a news release said.
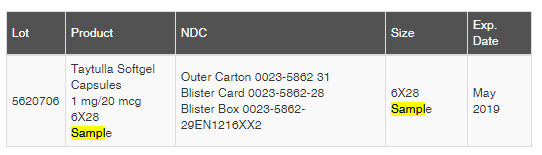
The Taytulla birth control pills are from lot 5620706 that expires on May 2019. They’ve been issued since Aug. 27, 2017.
Allegan is notifying customers by recall letter and is arranging for the return of the sample packs. Women who have the affected lot number should should notify their physician to arrange a return.
The FDA’s website has more information about the recall.
Consumers with questions about the recall are being asked to contact Allergan at 800-678-1605, Monday through Friday.







