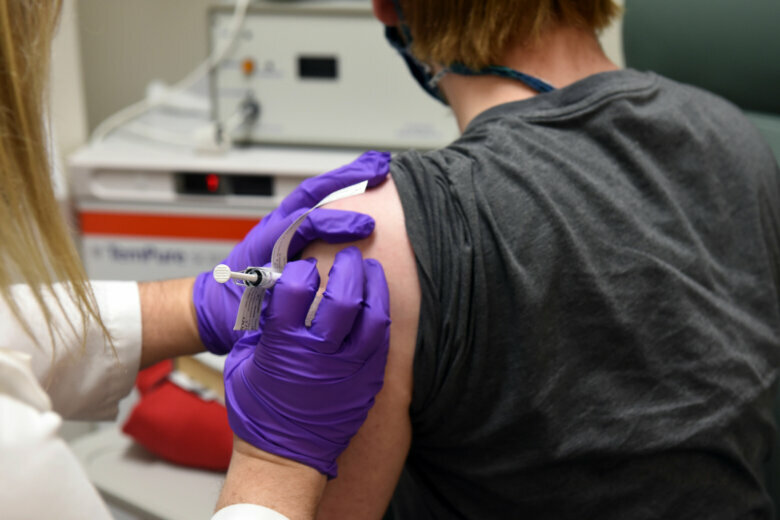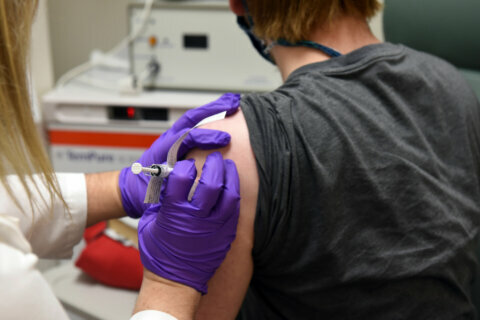
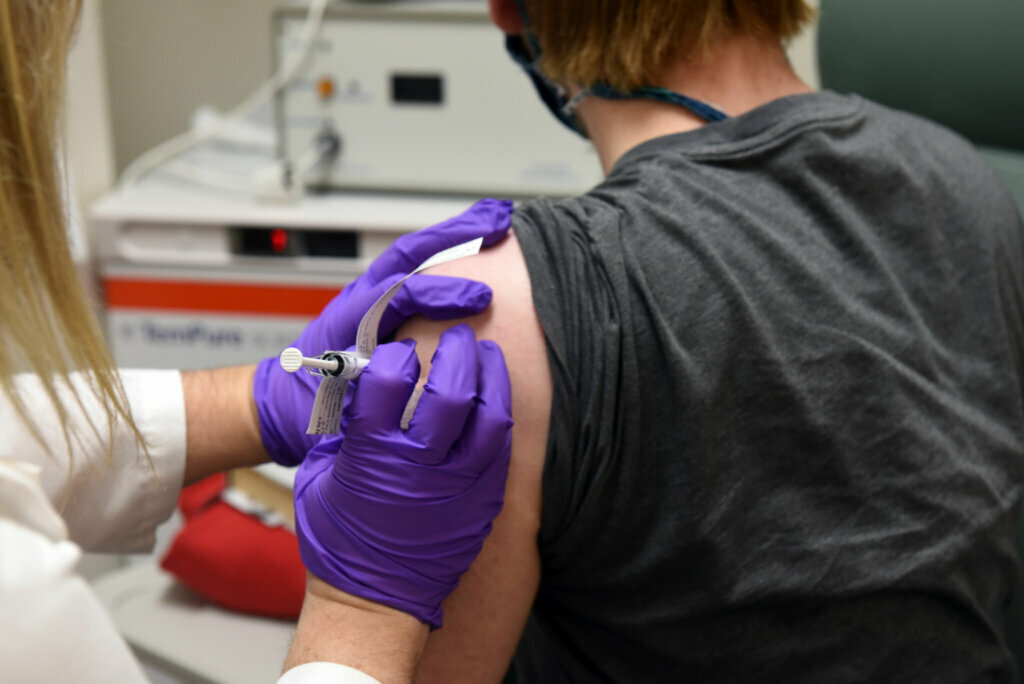
Researchers at the University of Maryland School of Medicine in Baltimore are taking part in an international trial on at least four experimental COVID-19 vaccines, and are seeking volunteers to take part.
David Rach, the first person to get the trial vaccine in Baltimore, told WTOP he was excited to be part of the study.
“They’ve moved from their animal trials and now they’re in phase one — and there always has to be a first person to go,” Rach said.
Some of Rach’s friends are worried about the risk of potential side effects, such as an allergic reaction. But Rach, who got the trial vaccine Monday, thinks participating is worth it.
“Actually being able to step up in [this] moment — it’s very exciting to be a part of,” Rach said.
- Sign up for WTOP alerts
- Latest coronavirus test results in DC, Maryland and Virginia
- Open up: Virginia dentists see nonemergency patients with measures in place
- Why another wave of coronavirus could hit in the fall, and how the US can stop it
- What happens if a coronavirus vaccine is never developed? It has happened before
- Coronavirus FAQ: What you need to know
- Coronavirus resources: Get and give help in DC, Maryland and Virginia
Rach is one of only about 360 participants in the entire study. In Baltimore, the trial will include up to 90 people.
“My mom’s proud; she’s currently calling all her friends,” Rach said.
Rach is a microbiology and immunology Ph.D. student at the University of Maryland School of Medicine, so he’s particularly keen to take part.
“This is my bread and butter; it’s areas that I’m interested in,” he said.
The trial vaccines differ from traditional inoculations in that they do not involve injecting viral proteins into a person’s body, the university said. Instead, each vaccine uses a different combination of messenger RNA to stimulate the production of protein antigens in a vaccinated person’s body that cause an immune response.
“A vaccine is urgently needed for COVID-19,” said School of Medicine Dean E. Albert Reece in a news release detailing the trials. “This research is an essential first step in protecting populations around the world from this serious illness.”
The vaccine candidates were jointly developed by U.S. drugmaker Pfizer and its German partner BioNTech, and the research is funded by Pfizer.
People participating in the trial will get two injections a month apart. Researchers will gather data on dosages and their impact on different age groups to determine which is best tolerated and produces the desired immune response.
Candidates to participate in the study need to be healthy and between ages 18 to 85.
The University of Maryland School of Medicine is still seeking participants in the trial. Those interested can visit the school’s website, text COVID19Vaccine to #555888, call 1-410-706-6156, or email clintrial@som.umaryland.edu for more information.