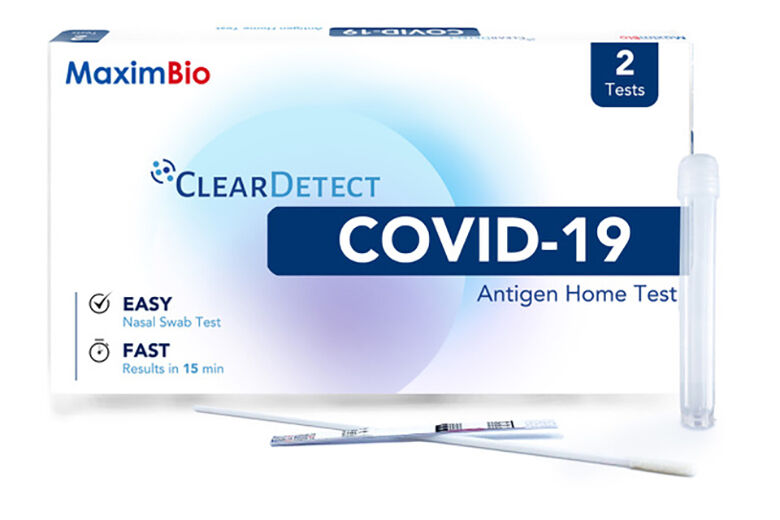
Rockville, Maryland-based Maxim Biomedical has received emergency use authorization from the Food and Drug Administration for its at-home COVID-19 antigen test called ClearDetect.
Unlike existing at-home COVID tests, it does not require an external reader or dropper bottles. It has three components: a swab; a test strip; and a test tube pre-filled with sample buffer.
A self-collected nasal swab is mixed with the sample buffer in the tube along with the test strip. It returns visual results as positive or negative within 15 minutes.
In a study with the National Institutes of Health using specimens positive for the omicron variant, it detected 100% of live virus, MaximBio said. When compared to an FDA-approved PCR method, the test achieved 86.9% positive accuracy, and 98.9% negative accuracy, it said.
MaximBio expects to begin production in late-February or early-March and will make the kits available packaged with either two tests in a box for at-home use, or bulk packs with 25 tests for healthcare, school and workplace settings. It did not say what the tests will cost, other than they will be competitively priced.
MaximBio has been working with NIH on the test as part of its Rapid Acceleration of Diagnostics program, or RADx. The research was funded in part by NIH and the Department of Health and Human Services.
MaximBio, founded in 2005, develops assay-based diagnostic tests. It has several tests or detecting HIV on the market.
- Sign up for WTOP alerts
- Latest coronavirus test results in DC, Maryland and Virginia
- Latest vaccination numbers in DC, Maryland and Virginia
Looking for more information? D.C., Maryland and Virginia are each releasing more data every day. Visit their official sites here: Virginia | Maryland | D.C.








