A pharmaceutical company is voluntarily recalling some birth control pills because they may be packaged in the wrong order or may have an empty blister pocket.
Apotex Corp. is recalling four lots of Drospirenone and Ethinyl Estradiol tablets USP, manufactured by Oman Pharmaceutical Products Co. LLC.
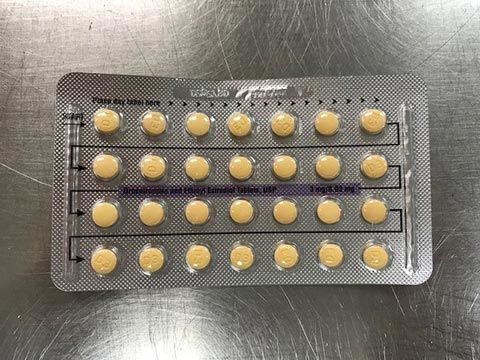
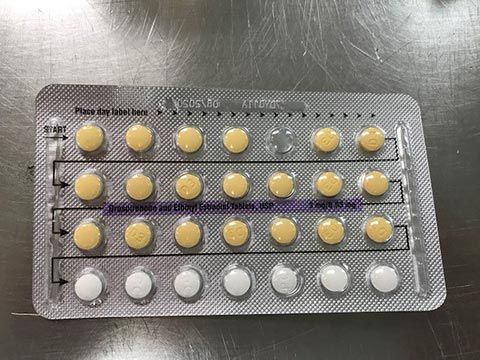
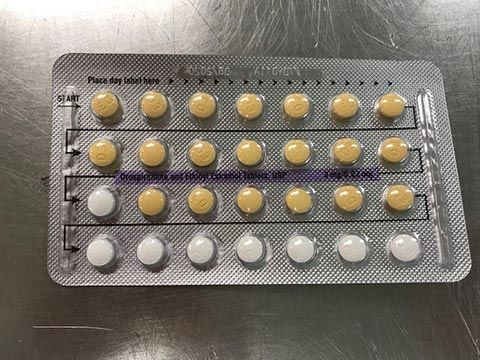
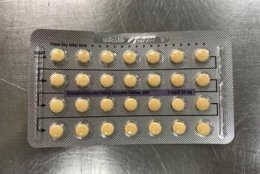
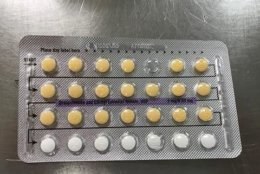
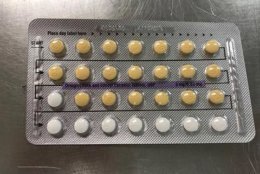
The affected tablet lots all expire in August 2020. The NDC number on the outer carton of the recalled product is 60505-483-3; the NDC number on the inner carton is 60505-4183-1. The outer carton contains three inner cartons; the inner carton contains one blister with 21 active, yellow color tablets and seven placebo, white color tablets.
The tablets are 3MG/0.03MG strength pills with the following lot number:
- 7DY008A
- 7DY009A
- 7DY010A
- 7DY011A
The Food and Drug Administration says the recalled birth control pills may be less effective if the patient does not take a tablet due to a missing pill or if a placebo is taken instead of an active pill.
Anyone taking the medication should continue using it, use a nonhormonal method of birth control and contact their health care provider for medical advice. Consumers may return the packages of affected medication to their pharmacist.
So far, there have been no cases of pregnancy or other adverse events reported to Apotex.
Adverse reactions or quality problems can be reported to the FDA’s MedWatch Adverse Event Reporting program.
Consumers with questions regarding the recall can call Apotex Corp at 1-800-706-5575 or email UScustomerservice@Apotex.com