During the global scramble to secure vaccines, many countries in Asia-Pacific were slow off the mark. This time, they’re not making the same mistake.
Countries around the region are rushing to place orders for the latest weapon against Covid-19: an antiviral pill that isn’t even authorized for use yet.
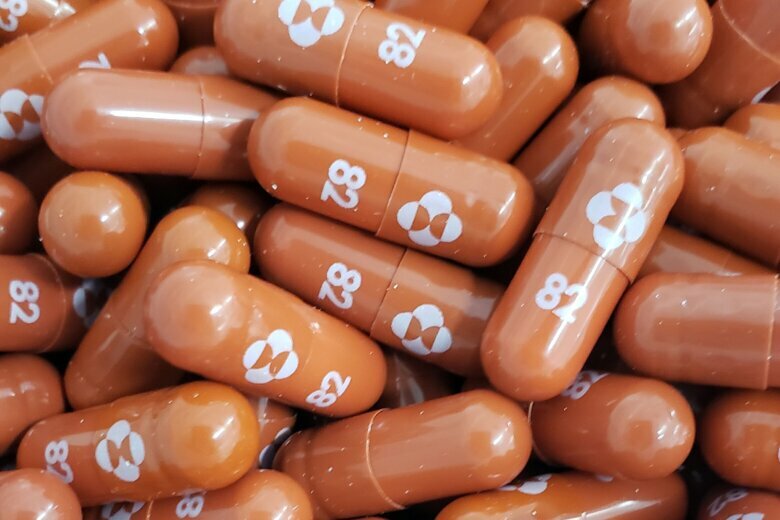
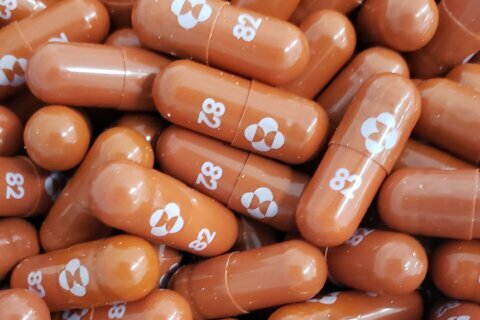
Molnupiravir — produced by US pharmaceutical company Merck — is being heralded as a potential pandemic game changer, especially for those unable to get vaccinated. Merck is seeking US Food and Drug Administration emergency use authorization for the drug — and if it’s granted, the capsule will become the first oral antiviral treatment against Covid-19.
Already, at least eight countries or territories in the Asia-Pacific region have signed deals or are in talks to procure the drug, according to analytics company Airfinity, including New Zealand, Australia and South Korea, all of which were relatively slow to start their vaccine programs.
Experts say while the pill looks promising, they worry some people will use it as an alternative to vaccines, which still offer the best protection.
And they caution that Asia’s race to stock up on the pill could see a repeat of the vaccine grab last year, when wealthier countries were accused of hoarding doses as lower-income countries missed out.
“(Molnupiravir) really does have the potential — the potential — to change the game a bit,” said Rachel Cohen, the North American executive director at non-profit Drugs for Neglected Diseases Initiative.
“We need to make sure that we don’t repeat history — that we don’t fall into the same patterns or repeat the same mistakes that we saw for Covid vaccines.”
What is molnupiravir?
Molnupiravir is seen as a positive step because it offers a way to treat Covid-19 — without patients needing to be in hospital.
The pill works like this: Once a patient is diagnosed with Covid-19, they can start a course of molnupiravir. That involves four 200-milligram capsules, twice a day, for five days — a total of 40 pills.
Unlike vaccines, which prompt an immune response, molnupiravir disrupts replication of the virus, said Sanjaya Senanayake, an infectious diseases physician and associate professor of medicine at Australian National University Medical School. “In a sense, it makes the virus produce unhealthy babies,” he said.
Interim Phase 3 results from a trial of more than 700 unvaccinated patients released earlier this month showed the pill might reduce the risk of hospitalization or death by approximately 50%, compared to patients who took a placebo. The participants were all given the pill or placebo within five days of symptom onset — and within 29 days, none of those who took the pill died, compared with eight who were given the placebo. Full data from the molnupiravir trial has not yet been released, and the data has not yet been peer-reviewed or published.
Wendy Holman, chief executive officer of Ridgeback Biotherapeutics, which is collaborating on the development, said in a statement the results were encouraging — and she hoped the drug could make a “profound impact in controlling the pandemic.”
“Antiviral treatments that can be taken at home to keep people with Covid-19 out of the hospital are critically needed,” she said.
Experts agree the drug is promising. Rather than patients waiting to see if they get seriously ill, the virus could potentially be treated straight after they are diagnosed, said Cohen, from the Drugs for Neglected Diseases Initiative.
And unlike other Covid-19 treatments, molnupiravir can be taken at home, freeing up hospital resources for more seriously ill patients.
“Getting a tablet is so much simpler,” Senanayake said. “This is a game changer.”
What the Covid pill means for vaccines
Vaccines are still the best protection, say experts — after all, they can reduce the risk of a person getting Covid-19 at all.
But even in Asia-Pacific, where vaccine rates in many countries have improved after a slow start, millions of people are still not inoculated either because they don’t qualify, or they can’t access shots.
And that’s where the pill comes in.
“There are lots of people that cannot get vaccinated,” said Nial Wheate, an associate professor at the University of Sydney’s School of Pharmacy. “This drug will be a frontline solution for those people that end up getting sick.”
But Wheate and other experts are concerned the pill may make it harder to convince some people to get vaccinated, compounding the vaccine hesitancy seen in a number of countries, including Australia.
Research shows people prefer to swallow medicines rather than be injected, Wheate said.
“If you’d said to me a year and a half ago that people will refuse a vaccine for a disease that’s wiping out the planet, I would have thought you’re crazy,” he said. “There is always scope for people to think that this drug will be a much better solution than getting vaccinated.”
But experts say the pill isn’t a replacement for vaccines.
Senanayake says the approach is similar to how we treat the flu — there’s a flu vaccine, but there are also antiviral medicines to treat those who become ill.
Cohen says the pill doesn’t mean there’s less urgency in scaling up equitable access to vaccines.
“Vaccine equity is sort of the defining challenge of our time. But you never fight an infectious disease with just one set of tools,” she said. “We really need the full arsenal of health technologies.”
Why Asia-Pacific countries are buying the Covid pill
According to Airfinity data, 10 countries or territories are in negotiations or have signed deals for the pill — and eight of them are in Asia-Pacific.
Some of those countries may be trying to avoid mistakes of the past when slow orders led to delayed vaccine rollouts.
“I think we just want to make sure that we’re ahead of the game when it comes to these other new developments,” Senanayake said.
“There’s a few middle-income countries in there that I think are just trying not to fall into the same trap that they were left in when high-income countries hoarded all the vaccines,” added Cohen.
It’s not clear how much each of these countries will pay for the pills.
The United States agreed to pay $1.2 billion for 1.7 million courses if the pill is approved, meaning the government is paying about $700 per course. An analysis by researchers Melissa Barber and Dzintars Gotham found that it costs about $18 to produce a course of molnupiravir based on a calculation of the cost of raw materials.
Gotham, who researches access to medicines, said it was common for pharmaceutical companies to impose a large markup on drugs, but said he was surprised to see that high a price since US funding contributed to the pill’s development.
Merck did not confirm whether those estimates were accurate, although in a statement to CNN, the company said the calculations don’t take into account research and development.
“We have not yet established a price for molnupiravir because it has not been approved for use,” the company said. “We have an advance purchase agreement with the US government and that price is specific to a substantial volume of molnupiravir and does not represent a list price for the US or any other country.”
In a statement in June, Merck said it planned to use a tiered pricing approach for different countries, and had also entered into licensing agreements with generic manufacturers to accelerate availability of the pill in 104 low- and middle-income countries.
A lack of equality
Lower-income countries may be at a disadvantage when it comes to using the pill.
Once the drug is approved for use, countries will need to decide whether to give it to anyone who shows symptoms, or to require a positive test before they can get it.
But that requires access to testing. And in some countries that could be an issue, said Cohen. The interim results on the pill are for people who were given it within five days of symptom onset — and in some countries, getting a test that quickly could be a problem.
Non-profit Doctors Without Borders hailed the drug as “potentially lifesaving care” for people living in areas where many are unvaccinated and vulnerable to the disease.
First, though, is the question of how they can access it.
While the drug would be simple to produce, according to Leena Menghaney, the South Asia head for the group’s access campaign, Merck controls the patent and is able to decide which countries to supply the drug to and at what price.
She renewed calls for a patent waiver that would waive intellectual property rights so that countries all over the world can produce versions of the medicine — potentially saving many more lives. Earlier in the pandemic, activists pushed for a waiver for Covid-19 vaccines, but the request was blocked by a small number of governments, including the United Kingdom.
Cohen said health tools and technologies should be treated as a public good — and that the situation raised questions about how we can make sure those benefits are shared equitably.
“We are concerned that that could potentially lead to a kind of therapeutic nationalism,” she said. “What we’re most concerned about, though, is that equitable access to antivirals may be particularly challenging in low- and middle-income countries.”
Senanayake said once again there was a risk of richer countries getting more than their fair share.
“With Covid, you have to be selfless to be selfish,” he said. “Otherwise, if you protect your own little cocoon, your own little country, if it occurs in other countries, then a new variant can emerge that can escape the vaccine.”