





WASHINGTON — A pharmaceutical company voluntarily recalled more bottles of infant ibuprofen this week over concerns they have an elevated concentration of the medicine.
It’s an expansion of a similar recall in December from Tris Pharma, which manufactured the medication for CVS, Walmart and Family Dollar stores.
Some of the lots, the company said, “have been found to contain Ibuprofen as high as 10 percent above the specified limit.” Safety for infants, they said, generally isn’t a concern until concentrations exceed 700 percent of the recommended dose.
The products affected by Tuesday’s recall and December’s recall include the CVS Health brand:
- Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 0.5 oz. bottle
- Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle
- Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
They also include the Equate brand, available in Walmart stores:
- Infants’ Ibuprofen Concentrated Oral Suspension, USP, 50 mg per 1.25 mL, in 1.0 oz. bottle
- Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
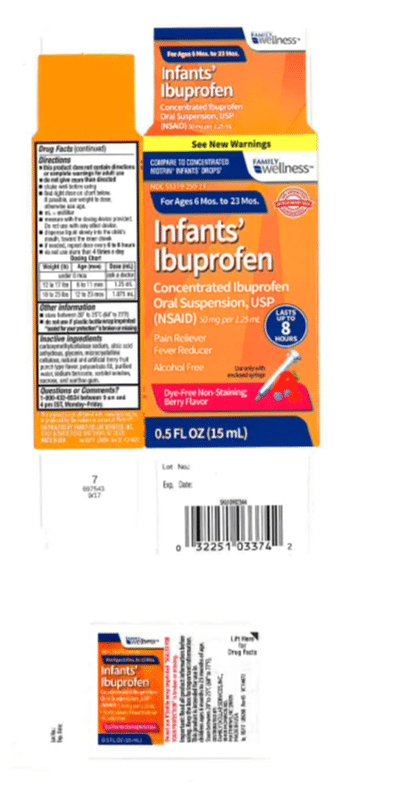
The affected bottles were also sold under the Family Wellness brand, available at Family Dollar Stores:
- Ibuprofen Oral Suspension Drops, USP, 50 mg per 1.25 ml, in 0.5 oz. bottle
Read more about the recall on Tris Pharma’s website.









